
In this section we will publish regular updates on stem cell and gene therapy.
Do you have any suggestions, let us know. Please click the icon.
newsflash:
induced neuronal cells

In this section we will publish regular updates on stem cell and gene therapy.
Do you have any suggestions, let us know. Please click the icon.
newsflash:
induced neuronal cells
gene therapy
stem cell and gene therapy
page last modified Jan 30th 2010
Wagner syndrome
developments
Stem cell and gene therapy might be the answer for people with retinal degenerative diseases like Wagner syndrome. The American government blocked this kind of research for a long time. At this time researchers from the UK are far ahead. At the UCL (University College London) Centre for Stem Cells and Regenerative Medicine there is a special team for ophthalmology research lead by professor Robin Ali.
Click Professor Robin Ali.pdf for a pdf with his profile.
About the research of him and his research group, he says on the website:
The main focus of my research is the development of gene and stem cell therapy with the primary aim of developing novel treatments for eye disease. Over the past ten years we have been optimising gene transfer to the eye. We are engaged in a comprehensive programme of work to develop gene therapy for eye disease and in particular for disorders affecting the retina, including inherited retinal degeneration as well as complex diseases such as those associated with retinal and choroidal neovascularisation and posterior uveitis. My research has utilised a variety of viral vectors, but my main interest is in the development of vector systems based on either adeno-associated virus (AAV) or lentiviruses. We have demonstrated the utility of these systems for gene transfer to the eye. A major aspect of our research is the development and use of a wide variety of genetic and experimental animal models of retinal disorders, including large animal models, in order to assess novel therapeutic approaches. We are engaged in a broad program of work to demonstrate proof of concept for a number of alternative strategies, including gene replacement therapy and/or delivery of siRNA to treat animal models of inherited retinal degeneration and delivery of genes encoding angiostatic, anti-apoptotic, immunomodulatory or neurotrophic molecules to treat a variety of animal models. We are now also investigating the potential of stem or progenitor cell transplantation to repair degenerating retinae. A recent key discovery is that transplantation of rod precursor cells at a specific stage of development results in their integration and subsequent differentiation into rod photoreceptors that form synaptic connections and improve visual function in mouse models of retinal degeneration. Conversely, transplantation of progenitor or stem cells that are not at this precise ontogenetic stage do not show this property and fail to integrate. We are now combining gene therapy approaches with that of stem cell transplantation and using viral vectors carrying genes encoding a variety of transcription factors in order to generate appropriate cells for transplantation from either embryonic or adult-derived stem cells. In order to deliver new treatments, we have now established a programme of translational research. My research group includes a number of clinicians (clinical training fellows as well as senior clinician/scientists) and we have strong links with a number of biotechnology companies. We have recently established a Department of Health funded clinical trial of gene therapy for a form of severe childhood-onset retinal dystrophy due to mutations in the gene encoding RPE65. This condition is likely to be particularly amenable to effective treatment and the trial should facilitate future trials and underpin the programme of development of treatments for other retinal disorders. The first patients were enrolled in early 2007 and the first results from the trial were published in the New England Journal of Medicine in May 2008.
Clic here for the original article.
stem cell therapy
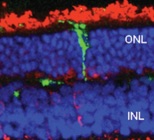
Müller stem cell transplantation in experimental models of retina degeneration. Following 2 weeks after transplantation of adult human Müller stem cells into the sub-retinal space of the dystrophic RCS rat, only a small number of transplanted cells (green) is able to migrate and integrate into the retina.
ONL, outer nuclear layer (photoreceptor cells)
INL, inner nuclear layer. Red staining indicates the expression of recoverin, a protein expressed by photoreceptor cells.
A very useful review article of from the UCL Institute of Ophthalmology, London is
Ocular regeneration by stem cells: present status and future prospects
G. Astrid Limb and Julie T. Daniels
British Medical Bulletin 2008;85: 47-61
DOI:10.1093/bmb/ldn008
In the clinical setting, stem cell transplantation to repair and regenerate human corneal epithelium has been successfully used for several years. The corneal surface is easy accessible. For complex tissues responsible for visual function, such as the neural retina and the retinal pigment epithelium (RPE), many problems to establish such therapies remain to be solved. Thus far the stem cell research for retinal regeneration is only done in animals.
Upon visual stimuli, all retinal neurons undergo a complex cascade of neural interactions through activation of synaptic pathways which finally lead to ganglion cells conveying visual messages via the optic nerve onto the brain. This complex process requires intact axonal connections, which are lost during retinal degeneration, and potential restoration of visual function critically depends on whether transplanted neurons can re-establish the dynamic neural network of the retina. Potential success of stem cell-based therapies may therefore depend on whether grafted cells can differentiate into the different retinal neurons, and whether these neurons can re-establish normal synaptic pathways within the host retina.
Neural cell death is the ultimate cause of blindness as a result of retinal degenerative diseases, including age-related macular degeneration (AMD), proliferative diabetic retinopathy (PDR), end-stage glaucoma, retinitis pigmentosa (RP), proliferative vitreoretinopathy and inherited retinal diseases.
The authors expect a lot of the RPE as a target tissue as it is a mono-cellular layer with a great impact on the visual stimuli. The RPE constitutes a mono-layer of pigmented cells attached to the inner surface of the Bruch’s membrane. It is directly positioned over the photoreceptor outer segments and plays an important role in retinal homeostasis. RPE dysfunction occurs in severe retinal diseases such as RP and AMD, for which stem cell transplantation to regenerate RPE cells may constitute a promising approach for treatment of these diseases.
Major advances in the development of stem cell therapies to regenerate RPE have been made during the last few years, with the findings that human embryonic stem cells provide a well-characterised and reproducible source of RPE that could potentially be used for human clinical studies.
(almost all text is from the article, as is the picture in the left column)
If you are interested, please email us at info@wagnersyndrome.eu
for a copy of this article using the code limb2008.
The review article of Gaillard (Poitiers, France) and Sauvé (Alberta, Canada) expresses serious doubts about the effectiveness of embryonic stem cells in degenerative retinal disorders.
Cell-based therapy for retina degeneration: The promise of a cure.
Frédéric Gaillard, Yves Sauvé
Vision Research, Volume 47, Issue 22, October 2007, Pages 2815-2824
Cell-based therapies in the retina have been associated with the recovery of visual function in animal models of retinal degeneration.
This review covers the current status of such therapies with regard to the source of the donor cells, their integration, and their impact on the degenerating host retina. Emphasis is also put on the importance of a careful interpretation of what is meant by ‘‘recovery of visual function’’. Two main approaches are considered here: (1) the use of human embryonic stem cell derived retinal pigment epithelial (RPE) cells to rescue photoreceptors in an animal model of RPE defect; and (2) the use of photoreceptor precursors to repair the degenerating neural retina. The current conclusions are that major hurdles have to be dealt with, such as finding an appropriate and ethically compliant donor cell source that would yield protracted survival and integration of the replacement retinal cells, and that there is no evidence yet that cell-based therapies can allow the long-term preservation or recovery of conscious vision. (abstract)
Gaillard and Sauvé start the article with some basics like what is vision? and the etiology of vision loss.
Optical images formed on the retina are transformed into neural images according to three sequential stages: (1) transduction of the light signals into electrical changes by the photoreceptors in the outer retina; (2) synaptic transmission of these electrical changes to cells in the inner retina; and (3) further synaptic transmission to retinal ganglion cells, the axons of which collect in the optic nerve, providing the only output to the brain.
Vision loss in most retinal degenerations is characterised by the progressive loss of photoreceptor cells initially affecting primarily the rods. The etiology is usually attributed to primary defects located either in the RPE cells (playing an essential role for photoreceptor function and survival) or the photoreceptors themselves.
The authors are less optimistic than others about the results of stem cell-based therapies for degenerative retinal disorders. They warn the reader to keep in mind that in order to be successful, cell therapy should fulfil four fundamental criteria. The desired cells should: (1) multiply in sufficient quantities, (2) organise into functional units, (3) survive and integrate into the host, and ultimately, (4) lead to the preservation of vision without causing deleterious side effects.
The most tested method (in animals) is to inject grafted human embryonic stem cell-derived RPE cells in the sub-retinal space. However it appears that they are incapable of forming a mono-layer over defective RPE. So the rescue of photoreceptor cells covers only the spot were the graft cells were injected. Secondly it causes a kind of complications related to retinal detachments: metamorphopsy and photoreceptor death.
To be successful in using human embryonic stem cell-derived RPE cells to rescue photoreceptors some crucial issues must be elucidated prior to considering clinical trials: the limited integration of the donor cells in the host, the mode of action of the donor cells (paracrine effect), the limitation of the RCS rat as a model of photoreceptor degeneration and relevant therapies, and last but not least must be considered if improving surgical techniques would not be a better option to preserve photoreceptor function.
The same goes for the use of photoreceptor precursors to repair the degenerating neural retina. And this method is even more complex than replacing the RPE. The hurdles the authors see in this respect are to establish other appropriate cell source, the optimal development stage of the donor cells, and some technical limitations as the enormous amount of cells that need to be injected in the sub-retinal space (40 times more than with RPE injections) that leads to even more severe effects on the retina (detachment-like) and how to prevent ongoing degenerative progress, that won’t be stopped by injecting photoreceptor precursors.
And we have to consider that saving RPE or photoreceptor cells in people with degenerative retinal disorders does not automatically mean that vision will be preserved or restored.
The authors conclude that looking for other possibilities than embryonic stem cells might have a more favourable future:
There is overwhelming evidence that optimal prevention of retinal degeneration (both anatomically and functionally) can be achieved with sub-retinal injections of cells that are readily available, safe, ethically acceptable and derived from sources other than from embryonic stem cells such as adult RPE cells line or adult syngeneic Schwann cells.
If you are interested, please email us at info@wagnersyndrome.eu
for a copy of this article using the code sauve2007.
A recent review article of Sander Smith et al from the UCL Institute of Ophthalmology, London, is
Prospects for retinal gene replacement therapy
Smith AJ, Bainbridge JW, Ali RR.
Trends Genet. 2009 Apr;25(4):156-65. Epub 2009 Mar 18.
The eye has several features that makes it particularly suitable as a target organ for gene therapy. The transparent nature of ocular tissues enables the visualization of vector delivery and the subsequent non-invasive imaging of transduced tissues. It is a small, compartmentalized and enclosed organ, enabling the delivery of small quantities of viral vector to particular subsets of ocular cell types with minimal risk of vector dissemination to the rest of the body. The blood-retinal barrier and blood-aqueous barrier maintain a degree of protection from immune responses directed against vector antigens that might otherwise cause inflammation and limit transgene expression. Finally, retinal function can be assessed using a variety of electrophysiological and psychophysical tests commonly used in the clinic.
However, the main conclusion from this article is that dominant inherited diseases like Wagners are unlikely to benefit from gene replacement therapies. Recessive disorders can -theoretically- be healed by gene replacement therapy through the delivery of normal copies of the defective gene via replication-deficient viral vectors. The normal copies only have to ‘conquer’ a recessive gene. For a dominant gene this concept is not very likely. The mutated dominant gene has to be repaired, and that is a complete other story.
If you are interested, please email us at info@wagnersyndrome.eu
for a copy of this article, using the code: smith2009.
This article refers to a website of the John Moran Eye Center, University of Iowa that provides in-depth knowledge of the retina. With beautiful images like the one below.
Please see links
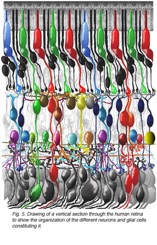
drawing of a vertical section through the human retina to show the organization of the different neurons and glial cells constituting it